
Place, publisher, year, edition, pages2009. 2009, 74, 118), whereas carba-LNA-6', 5'-phosphodiester modification destabilizes both AON/RNA and AON/DNA significantly (by -10 to -19 degrees C/modification), which, as shown in our comparative CD studies, that the cyclic phosphotriester modified AONs as well as carba-LNA-6'.5'-phosphodiester modified AONs are much more weakly stacked than carba-LNA-3',5'-phosphodiester-modified AONs. It is noteworthy that Carba-LNA-3',5'-phosphodiester modification stabilized the AON/RNA duplex by +4 degrees C/modificafion (J. 9 many different types of backbone modifications have been reported for sirnas.
#Phosphate backbone plus#
Treatment of (Sp)-D-2-CNA-modified AON with concentrated aqueous ammonia gave cwba-LNA-6',5'-phosphodiester modified AON (similar to 80%) plus a small amount of carba-LNA-3',5'-Phosphodiester-modified AON (similar to 20%). the natural negatively charged phosphodiester backbone of rna is beset with poor cell-membrane permeability and is a substrate for ubiquitous ribonucleases, which rapidly degrade the rna to monomers and/or short oligomers. This showed that this cyclic phosphotriester modification destabilizes the AON/DNA and AON/RNA duplex by about -6 to -9 degrees C/modification. The effect of pure (S-p)-D-2-CNA niodification in the AONs was estimated by complexing to the complementary RNA and DNA strands by the thermal denaturation studies. Thus, after removal of the solid supports from the (S-p)-D-2-CNA-modified AON by BDU/MeCN, they were treated with hydrazine hydrate in pyridine/AcOH to give pure AONs in 35-40% yield, which was unequivocally characterized by MALDI-TOF to show that they have an intact six-membered dioxaphosphorinane ring. In contrasts the (S-p)-D-2-CNA was about 2 times more stable than (Rp)-D2-CNA under hydrazine hydrate/pyficfine/AcOH (pH = 5.6), which was exploited in the deprotection of pure (S-p)-D-2-CNA incorporated antisense oligodeoxynucleotides (AON). Thus, both (Sp)- and (Rp)-D2-CNA dimers (17a and 17b) were very labile toward nucleophile attack in concentrated aqueous ammonia to give carba-LNA-6',5'-phosphodiester (21) approximate to 70-90%, carba-LNA-3',5'-phosphodiester (22) approximate to 10%, and carba-LNA-6',3'-phosphodiester (23) < 10%. The chemical reactivity of the six-membered dioxaphosphorinane ring in D-2-CNA was found to be dependent on the internucleotidic phosphate stereochemistry. It turned out that at equilibrium concentration the (S-p)-D-2-CNA isomer is preferred over the (R-p)-D-2-CNA isomer by 0.39 kcal/mol. It has been found that F- ion can catalyze the isomerization of pure (S-p)-D-2-CNA or (R-p)-D-2-CNA to give an equilibrium mixture (K = 1.94). Structural studies by NMR as well as by ab initio calculations showed that in (S-p)- and (R-p)-D-2-CNA the Mowing occur: (i) the sugar is locked in extreme North-type conformation with P = 11 degrees and Phi(m) (ii) the six-membered 1,3,2-dioxaphosphorinane ring adopts a half-chair conformation (iii) the fixed phosphate backbone delta, epsilon, and zeta torsions were found to be delta, epsilon (cis), zeta for (S-p)-D-2-CNA, and delta , epsilon(cis), zeta for (R-p)-D-2-CNA. Two diastereomerically pure carba-LNA dioxaphosphorinane nucleotides , simultaneously conformationally locked at the sugar and the phosphate backbone, have been designed and synthesized. 3248-3265 Article in journal (Refereed) Published Abstract
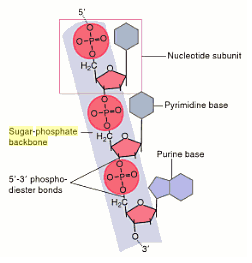
The sugar-phosphate backbone is negatively charged due to the presence of the phosphate groups hence making it also hydrophilic.
#Phosphate backbone skin#
The phosphate group always sticks out of the double helix of DNA due to the arrangement of the bases within the helix, and because phosphate groups are found throughout the entire DNA molecule, sticking outside the helix, it is referred to as a 'phosphate backbone' (imagine the bones that protrude through the skin along your spine). This is formed by the repeating sequence of a phosphate group and deoxyribose, connected in sequence by phosphodiester bonds. A phosphate backbone is the fixed, structural feature from which nucleic acids protrude in DNA and RNA.


 0 kommentar(er)
0 kommentar(er)
